Giant Axonal Neuropathy (GAN)
Giant Axonal Neuropathy (GAN) is an extremely rare, terminal neurodegenerative disorder. It generally appears in early childhood, (onset is typically 2.5 years of age). It progresses slowly as neuronal injury becomes more severe. Signs of GAN usually begin in the peripheral nervous system, which controls movement and sensation in the arms, legs, and other parts of the body. Most individuals with this disorder first have problems with walking. Later they may lose sensation, coordination, strength, and reflexes in their limbs. Hearing and visual problems may also occur. Extremely kinky hair (as compared to siblings) is characteristic of giant axonal neuropathy.
Giant axonal neuropathy (GAN) is an inherited condition involving dysfunction of a specific type of protein in nerve cells (neurons). The protein is essential for normal nerve function because it forms Neurofilaments make up a structural framework that helps to define the shape and size of the neurons. This condition is characterized by abnormally large and dysfunctional axons, which are the specialized extensions of nerve cells that are required for the transmission of nerve impulses.
Presently there are less than 100 diagnosed cases of GAN in the world. Currently, there is no cure or treatment for GAN. As the disorder progresses, patients become quadriplegics, dependent on a feeding tube and ventilator. Children who are diagnosed with the disease are not expected to live beyond their early twenties, while some die much younger.
How do people get Giant Axonal Neuropathy?
This condition is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically don’t show signs and symptoms of the condition.
Research Efforts:
A portion of the donations we receive are allocated to support the groundbreaking gene therapy research of Dr. Steven Gray at UT Southwestern Medical Center in Dallas, Texas. An ongoing human clinical trial at the NIH seeks to use gene therapy to halt the progression of the disease. If successful, gene therapy holds the potential to treat hundreds of childhood neurological diseases like GAN.
Gene Therapy
Gene therapy is an experimental technique that uses genes to treat or prevent disease. In the future, this technique may allow doctors to treat a disorder by inserting a gene into a patient’s cells instead of using drugs or surgery. Researchers are testing several approaches to gene therapy, including:
- Replacing a mutated gene that causes disease with a healthy copy of the gene.
- Inactivating, or “knocking out,” a mutated gene that is functioning improperly.
- Introducing a new gene into the body to help fight a disease.
Although gene therapy is a promising treatment option for a number of diseases (including inherited disorders, some types of cancer, and certain viral infections), the technique remains risky and is still under study to make sure that it will be safe and effective. Gene therapy is currently being tested only for diseases that have no other cures.
Gene therapy is designed to introduce genetic material into cells to compensate for abnormal genes or to make a beneficial protein. If a mutated gene causes a necessary protein to be faulty or missing, gene therapy may be able to introduce a normal copy of the gene to restore the function of the protein.
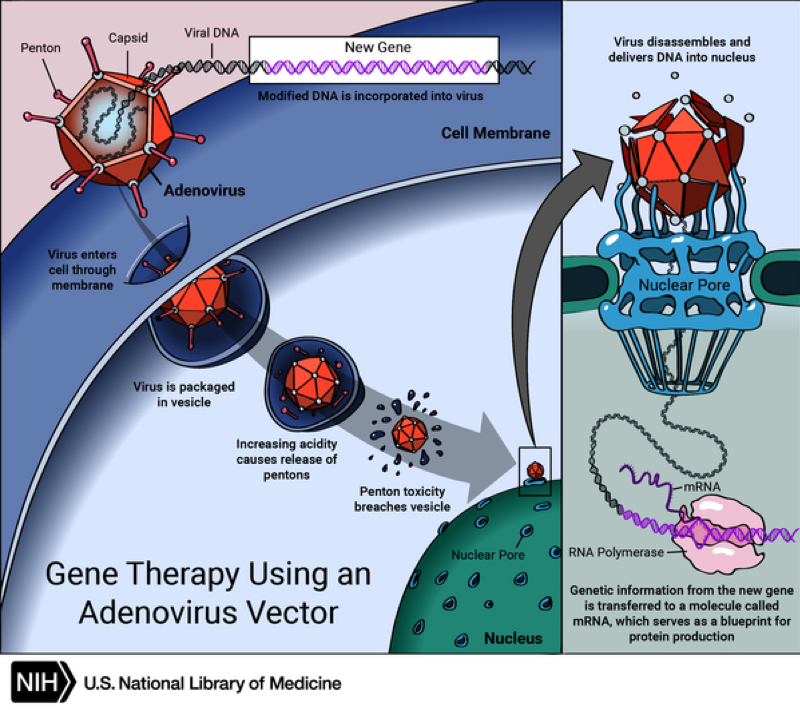
A gene that is inserted directly into a cell usually does not function. Instead, a carrier called a vector is genetically engineered to deliver the gene. Certain viruses are often used as vectors because they can deliver the new gene by infecting the cell. The viruses are modified so they can’t cause disease when used in people. Some types of virus, such as retroviruses, integrate their genetic material (including the new gene) into a chromosome in the human cell. Other viruses, such as adenoviruses, introduce their DNA into the nucleus of the cell, but the DNA is not integrated into a chromosome.
The vector can be injected or given intravenously (by IV) directly into a specific tissue in the body, where it is taken up by individual cells. Alternately, a sample of the patient’s cells can be removed and exposed to the vector in a laboratory setting. The cells containing the vector are then returned to the patient. If the treatment is successful, the new gene delivered by the vector will make a functioning protein.
Researchers must overcome many technical challenges before gene therapy will be a practical approach to treating disease. For example, scientists must find better ways to deliver genes and target them to particular cells. They must also ensure that new genes are precisely controlled by the body.

